Product Development
Artemida Pharma provides independent input to help our clients produce a robust Development Strategy. Our consultants have extensive industry experience and deliver tailored solutions that are cost and time sensitive.
Our Approach
- Define project needs: detailed review and evaluation with client
- Anticipation of future requirements of project
- Identification of tailored solutions
- Optimisations of stategy
- Selection of supplier based on project needs
- Rapid set-up
- Dedicated Project Lead providing efficient & effective management
- Ongoing communication
- Flexible resources & expertise to fulfil specific project requirements
- Optimisations of stategy
- Selection of supplier based on project needs
- Rapid set-up
- Dedicated Project Lead providing efficient & effective management
- Ongoing communication
- Flexible resources & expertise to fulfil specific project requirements
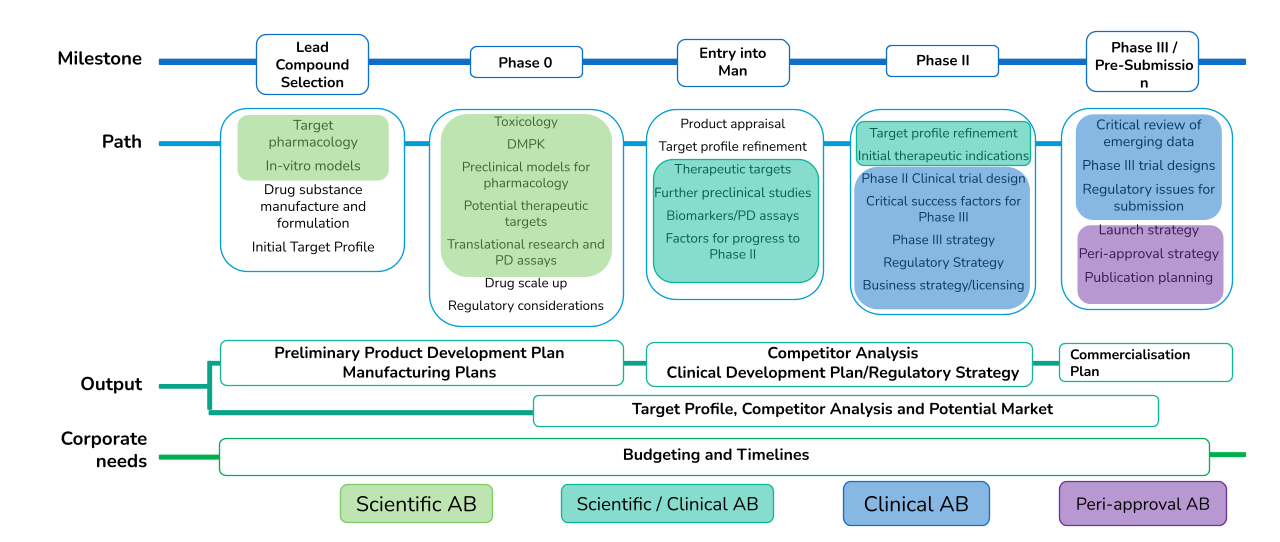
- Target phamacology
- In-virto models
- Drug substance manufacture and formulation
- Initial Target Profile
- Toxicology
- DMPK
- Pleclinical models for pharmacology
- Potential therapeutic targets
- Traditional research and PD assays
- Drug scale up
- Regulatory considerations
- Product appraisal
- Target profile refinement
- Therapeutic targets
- Further preclinical studies
- Biomarkers / PD assays
- Traditional research and PD assays
- Target profile refinement
- Initial therapeutic indications
- Phase II Clinical trial design
- Critical success factors for Phase III
- Phase III strategy
- Regulatory Strategy
- Business strategy / licensing
- Critical review of emerging data
- Phase III trial designs
- Regulatory issues for submission
- Launch strategy
- Peri-approval strategy
- Publication planning
- Preliminary Product Development Plan
- Manufacturing Plans
- Competitor Analysis
- Clinical Development Plan/Regulatory Strategy
- Commercialisation Plan
- Target Profile, Competitor Analysis and Potential Market
- Budgeting and Timelines
Artemida Pharma provides support across the spectrum of drug development from pre-clinical research to entry-into-man studies to peri-approval. Whether it be the initial scientific steps, the intersection of the science with clinical milestones, or post-approval strategies, we help our clients choose the optimum route through the development pathway to suit their needs.
Partnerships in drug development vary according to the stage of development, with early stages attracting government and philanthropic organisations, compared to later stages typically attracting pharma or venture capitalists. Our resources, network and expertise at Artemida Pharma result in a tailored approach to linking our clients with investors. We carefully plan the scope of a deal including scenarios for success, ensuring we create the best possible strategy for a profitable return on investment.
We design and prepare project plans to maximise your product asset value. The first step is a high-level evaluation, involving the review of existing data and the execution of gap analyses to curate a bespoke strategic plan. Potential partners are then assessed, allowing optimal market positioning. Finally, we carry out an in-depth data review plan with next step recommendations from a business perspective to ensure a successful return on investment.
Artemida Pharma has developed a strong network of licensing partners through our previous experience, conference partnerships, database access and office co-location with large pharmaceutical companies. We are an independent company with a strong reputation in this area and work with our clients to help them negotiate successful partnerships
Our approach to out-licensing partnering involves:
- High level evaluation to support mechanism of and candidate profile of the therapeutic product
- Detailed appraisal and analysis of data to finalise approach for candidate, and to support deal content and structure